NSQ (Not of Standard Quality) alerts stem from random monthly sample testing conducted
by state drug authorities.
Several pharmaceutical companies, including Hetero Drugs, Alkem Laboratories, Hindustan
Antibiotics Limited (HAL), Karnataka Antibiotics & Pharmaceuticals Ltd, Meg Lifesciences,
Pure & Cure Healthcare, and others, have been flagged in the report.
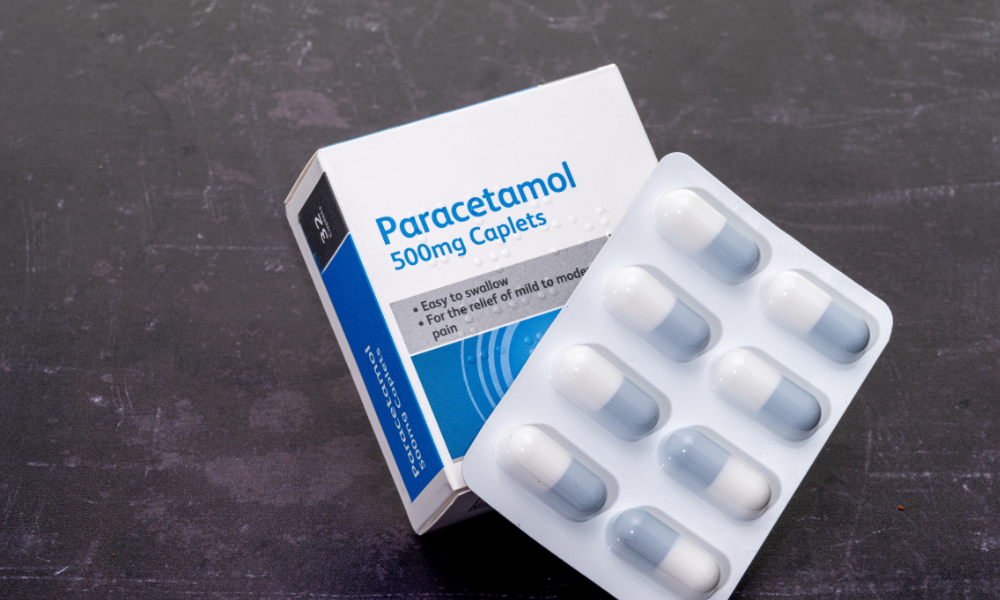
New Delhi: The Central Drugs Standard Control Organisation (CDSCO) recently identified
over 50 drugs that do not meet the required standards, with Paracetamol among them.
In its latest monthly drug report, the CDSCO revealed that various medicines, including
Paracetamol tablets, calcium and vitamin D3 supplements, anti-diabetic medications, and
blood pressure control drugs, have failed to meet quality requirements.
Paracetamol tablets produced by Karnataka Antibiotics & Pharmaceuticals Ltd were among
those flagged for quality concerns.
According to the report, a total of 48 medicines were classified as “not of standard quality.”
Unexpectedly, a Gauze Roll Non-Sterile Roller Bandage was also found to fall within this
category.
The drug regulator also released an additional list of five drugs that failed quality tests, along
with responses from the pharmaceutical companies involved. Interestingly, some companies
refused to take responsibility for the flagged drugs, claiming that the products in question
were counterfeit.
One of the responses from a manufacturing firm indicated, “The actual manufacturer (as
claimed on the label) has stated that the batch in question was not produced by them, and the
product is counterfeit. This claim is pending further investigation.”